Abstract
Aims/hypothesis
Prevention trials in first-degree relatives of type 1 diabetic patients are hampered by large interindividual differences in progression rate to diabetes. We investigated whether specific combinations of immune and genetic markers can identify subgroups with more homogeneous progression to clinical onset.
Methods
Antibodies against islet cell cytoplasm (ICA), insulin (IAA), glutamate decarboxylase (GADA) and IA-2 protein (IA-2A) were measured in 790 non-diabetic control subjects and 4,589 first-degree relatives under age 40.
Results
On first sampling, 11.1% of the siblings presented at least one antibody type (p<0.001 vs other relatives). During follow-up (median 52 months) 43 subjects developed type 1 diabetes (31 siblings, ten offspring of a diabetic father, two offspring of a diabetic mother). Using Kaplan–Meier survival analysis and Cox regression, IA-2A conferred the highest 5-year diabetes risk (>50%) irrespective of the number of antibodies present. In initially IA-2A-positive relatives (n=58) progression to hyperglycaemia depended more on HLA DQ status than on type of kinship (84% progression in the presence of DQ2/DQ8 vs 32% in its absence; p<0.003). In IA-2A-negative relatives (n=4,531) 5-year progression to diabetes increased with the number of other antibodies (ICA, GADA and/or IAA) (p<0.001) but overall did not exceed 10% even for two or more antibodies. Among relatives initially positive for one or more antibody type other than IA-2A (n=315), there was significantly more progression to diabetes (overall still <10%) in carriers of DQ2 (p<0.001 vs no DQ2), regardless of DQ8 status.
Conclusions/interpretation
These observations suggest that the HLA-DQ-inferred risk of diabetes can proceed through two distinct pathways distinguished by IA-2A status. Combined positivity for DQ2/DQ8 and IA-2A defines a more homogeneous high-risk population for prevention trials than those used so far.
Similar content being viewed by others
Introduction
The appearance of circulating islet autoantibodies before the clinical onset of immune-mediated type 1 diabetes makes it possible to select subjects at increased risk of becoming hyperglycaemic among first-degree relatives of known patients [1–9]. However, prevention studies testing the potential of pharmacological interventions to arrest or slow down the subclinical disease process in antibody-positive relatives are still hampered by large differences in disease progression rate in marker-positive subjects [1–3, 10–12]. Therefore, the study design should benefit from further refining the inclusion criteria in order to select participants with a more homogeneous risk of the disease, thereby reducing the numbers needed to treat [3, 12].
A recent study in siblings of type 1 diabetic patients [13] confirmed the generally held view that the risk of diabetes increases with the number of islet autoantibody specificities [4–9], but in addition indicated that the presence of autoantibodies against the intracellular domain of insulinoma-associated protein-2 (IA-2 autoantibodies, IA-2A), which is often associated with multiple antibody positivity [13–16], confers a higher risk of rapid progression towards clinical onset than multiple antibody positivity per se [13]. In persistently IA-2A-negative siblings the risk of diabetes increased significantly with the number of other autoantibodies present (GADA, IAA and/or ICA), but the progression rate remained less than 10% within 5 years [13]. These findings warrant a search for additional biological markers that may complement autoantibody measurements for refining diabetes risk assessment. In this respect genetic markers should be considered, especially positivity for HLA DQA1*0301-DQB1*0302 (DQ8) and HLA DQA1*0501-DQB1*0201 (DQ2) risk haplotypes, alone or in heterozygous combination (DQ2/DQ8), the latter being preferentially associated with early-onset diabetes [17, 18].
Since type 1 diabetes prevention studies have included types of first-degree relatives apart from siblings, and sometimes even second-degree relatives [10, 11], it is also important to test the predictive value of marker combinations in different types of first-degree relatives, who have been reported to differ in terms of their disease risk [19, 20]. We therefore measured IAA, ICA, GADA and IA-2A yearly in a large group of siblings, offspring and parents of type 1 diabetic patients, and followed them in terms of diabetes development to investigate whether (1) the frequency and diabetes-predictive value of the various islet autoantibodies tested, alone or in combination, varied according to the type of kinship to the proband; (2) the presence of HLA DQ risk haplotypes or genotypes could influence the progression rate to diabetes in antibody-positive relatives and explain (part of) the differences among relatives; and (3) specific combinations of HLA DQ haplotypes or genotypes and antibody profiles could further refine risk assessment.
Subjects and methods
Subjects and data collection
Siblings (n=1,727), offspring (n=2,379) and parents (n=483) of type 1 diabetic patients were consecutively recruited by the Belgian Diabetes Registry (BDR) between 1 January 1989 and 31 December 2001 according to previously defined criteria [13, 21]. At entry, blood was sampled and a short questionnaire with demographic, familial and personal information was completed. The relatives were not prescreened for ICA or any other autoantibody before entering the study. The group of first-degree relatives (n=4,589), aged 0–39 years, was followed for a median (interquartile range) period of 52 (43–59) months. The predictive value of biological markers was based on the results of baseline samples. The non-diabetic control subjects (n=790), aged 0–39 years, were recruited among blood donors, laboratory personnel and children attending wards for minor surgery, including correction of phimosis. None of the controls’ relatives had type 1 diabetes. Overall, there was no difference in age between relatives and the control group, but on average siblings were younger than parents (p<0.001) and older than offspring (p<0.001) (Table 1). BMI was recorded and analysed after transformation into a standard deviation score (SDS) in comparison with data from a reference cohort comprising 15,636 male and 14,899 female subjects recruited between 1978 and 1990 [22, 23]. Relatives who developed diabetes during follow-up were identified through repeated contacts with Belgian endocrinologists and paediatricians, self-reporting through yearly questionnaires and a link with the BDR patient database, where newly diagnosed diabetic patients under age 40 are registered. All participating first-degree relatives gave informed consent and the study was approved by the ethics committees of BDR and participating university hospitals and was carried out in accordance with the Declaration of Helsinki as revised in 2000 (http://www.wma.net./e/policy/b3.htm).
Assays
ICA were determined by indirect immunofluorescence and endpoint titres expressed as Juvenile Diabetes Foundation (JDF) units [24]. IA-2A, GADA and IAA were measured by liquid-phase radiobinding assays and expressed as percentage of tracer bound in haemolysis-free sera [24]. Cutoff values for antibody positivity were determined as the 99th percentile of antibody levels obtained in 790 non-diabetic control subjects after omission of outlying values, and were ≥12 JDF units for ICA, ≥0.6% for IAA, ≥2.6% for GADA and ≥0.4% for IA-2A [24]. Between-day coefficients of variation determined on human control sera were 11% (n=474) for IA-2A, 10% (n=427) for GADA and 9% (n=413) for IAA at the level of 1.8, 7.8 and 7.2% tracer binding, respectively. In the IDW Combinatorial Workshop [25] diagnostic sensitivity adjusted for 99% specificity was 73% for ICA, 85% for GADA and 36% for IAA. In the DASP 2002 [26], sensitivity and specificity were 36 and 98% for IAA, 88 and 96% for GADA, and 62 and 97% for IA-2A, respectively. cDNAs for the preparation of radiolabelled GAD and the intracellular domain of IA-2 (amino acids 603–980) were kindly donated by Professor Å. Lernmark (University of Washington, Seattle, WA, USA) and Dr. M. Christie (King’s College School of Medicine and Dentistry, London, UK), respectively. All initially antibody-positive relatives (n=373) as well as the vast majority of the entire group (3,780/4,589) were genotyped for HLA DQA1 and DQB1 as previously described [18]. Plasma glucose was measured as before [13].
Statistical analysis
Statistical differences between groups were assessed with the Chi-square test with Yates’ correction or Fisher’s exact test for categorical variables, and with the Mann–Whitney U-test for continuous variables. Diabetes-free survival was estimated by Kaplan–Meier analysis [27] and differences in progression to diabetes with the log–rank test [28]. The Cox proportional hazards model, performed by the forward stepwise method, was used to investigate the independent contributions of risk factors identified by univariate analysis, with calculation of 95% confidence intervals as hazard ratios [29]. All statistical tests were performed two-tailed using SPSS for Windows 11.0 (SPSS, Chicago, IL, USA) and considered significant whenever p<0.05 or, in case of k comparisons, whenever p<0.05/k (Bonferroni adjustment) [30].
Results
Autoantibody positivity
At first sampling, 4% of siblings tested positive for ICA, which tended to be higher than in offspring of a diabetic father, and was significantly higher than in offspring of a diabetic mother, in parents and in control subjects (overall p<0.001; Table 2). The hierarchy of antibody positivity according to kinship was similar for the other individual antibodies as well as for the number of antibodies (Table 2). Within each category of relatives the highest frequencies were noted for GADA and IAA, and the lowest for IA-2A (Table 2). Overall, 11.1% of siblings (p<0.001 vs all other subject groups) and 7.1% of offspring of a diabetic father presented at least one type of antibody at first sampling. In siblings and offspring there were no differences in antibody frequency according to age (0–9 vs 10–39 years), except for IAA, for which the frequency was significantly higher at younger ages (not shown).
Progression to type 1 diabetes
During follow-up, 31 siblings, ten offspring of a diabetic father, two offspring of a diabetic mother and none of the parents developed type 1 diabetes with a progression frequency of, respectively, 18, 8, 2 and 0‰ (p<0.001) and a median (interquartile range) time to diabetes of 28 (9–44) months. Their median (interquartile range) age at diagnosis was 13 (10–20) years, with a male/female ratio of 23:20 (1.15). The various types of prediabetic relatives did not differ significantly overall in demographic and genetic parameters or in median time to diabetes (not shown).
Progression to diabetes was significantly associated with antibody positivity, especially IA-2A, and increased with the number of autoantibodies present at first sampling with (not shown) or without (Table 3) inclusion of ICA (p<0.001). However, when considering possible combinations of two molecular antibodies (n=70), progression was significantly higher in the presence than in the absence of IA-2A (p<0.001) (Table 3).
Initially IA-2A-positive relatives (n=58)
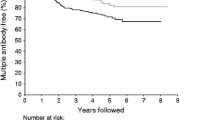
Although siblings and offspring of a diabetic father tended to develop more diabetes than offspring of a diabetic mother or parents, there was no significant difference in risk according to kinship (overall p>0.05; not shown). However, when all IA-2A-positive relatives were analysed according to HLA DQ risk status, significantly more subjects progressed to diabetes in the presence than in the absence of the DQ8 susceptibility haplotype (p=0.038). Especially the DQ2/DQ8 genotype (n=17) was associated with the highest 5-year progression rate [95% confidence interval] towards diabetes (84% [65–100%] vs 32% [16–48%] for all other genotypes (n=41), p=0.003; Fig. 1). This result was confirmed in the group positive for IA-2A and IAA or IA-2A and GADA (p<0.001; Table 3).
Diabetes-free survival in IA-2A-positive relatives (n=58) after stratification for all possible combinations of the presence or absence of both risk haplotypes, HLA DQ2 and HLA DQ8 (DQ2/non-DQ8 [— - — -] vs nonDQ2/DQ8 [- - - -] vs nonDQ2/nonDQ8 [····] vs DQ2/DQ8 [—]). In each panel the 5-year diabetes-free survival (95% confidence interval) is shown for each arm.
Initially IA-2A-negative relatives (n=4,531)
Progression to diabetes within 5 years was significantly associated with the number of other molecular antibodies at initial sampling (overall p<0.001), but did not exceed 10% even in the presence of IAA and GADA (Table 3). Similar results were observed when also considering ICA (not shown). Within the IA-2A-negative relatives positive for ICA, GADA and/or IAA (n=315), there was no significant difference in progression rate according to the type of kinship (not shown). In contrast, with the IA-2A-positive subjects progression to diabetes did not differ according to DQ8 status (not shown), but there was significantly more progression [95% confidence interval] in carriers of DQ2 (8% [4–13%]) than in relatives without DQ2 (1% [0–3%]; p<0.001).
Characteristics of initially antibody-positive relatives
After stratification for IA-2A status, prediabetic and non-diabetic relatives did not differ significantly in terms of age, male/female ratio, SDS-BMI, type of relationship, random glycaemia, or antibody frequency and levels (in case of antibody positivity) at first sampling.
HLA DQ risk haplotypes and genotype according to type of relationship
All relatives under age 20 years (n=2,812) were HLA-DQ-genotyped and stratified according to the type of relationship. Siblings carried DQ2 (46 vs 39%; p=0.002), especially the high-risk DQ2/DQ8 genotype (12 vs 6%; p<0.001), more frequently than offspring, regardless of the gender of the diabetic patient. Siblings and offspring did not differ significantly in DQ8 prevalence (35 vs 33%).
Cox regression analysis
Univariate analyses (enter method) were first performed in which each of the potential risk factors for diabetes was confirmed to be significantly associated with progression to diabetes (Table 4). To assess the independent contributions of these identified biological predictors, multivariate forward stepwise Cox regression analysis was carried out. In a first multivariate model including all genotyped relatives (n=3,780), the number of detected antibodies, IA-2A, HLA DQ2/DQ8 and being a sibling contributed independently to the risk of diabetes (Table 4). Next, we investigated the hazard ratio for diabetes of the parameters selected in model 1 in the initially antibody-positive subjects at increased risk (model 2). Only IA-2A and HLA DQ2/DQ8 were selected as significant independent predictors (p<0.001) (Table 4). Similar results were obtained when ICA was omitted from the analysis (not shown).
Discussion
In the present study in 4,589 first-degree relatives of type 1 diabetic patients analysed at study entry for diabetes autoantibodies (IAA, ICA, GADA, IA-2A) and HLA DQ genotype and followed for several years, the frequency of individual or multiple autoantibody positivity and progression to diabetes tended to be graded according to the known hierarchy of risk among first-degree relatives (siblings > offspring diabetic father ≫ offspring diabetic mother ≅ parents) [19, 20]. In relatives with circulating autoantibodies at study entry, the progression rate to diabetes depended much more on HLA DQ genotype than on the type of relationship to the proband. Differences in frequency of HLA DQ risk haplotypes or genotypes could partially explain differences in progression rates between siblings and offspring, but not differences between offspring of a diabetic father and offspring of a diabetic mother.
The present study also confirmed that IA-2A signals a higher risk of impending clinical onset of diabetes than multiple antibody positivity per se [13]. IA-2A positivity at study entry conferred greater than 50% risk of diabetes within 5 years regardless of the number of other antibodies present or the type of relationship to the proband. In the absence of IA-2A, other antibodies are also predictive of diabetes but to a much lower degree. Although the number of relatives included and their follow-up time may be smaller than in some other reports [6, 9], the present study has analysed a large unselected group of first-degree relatives with a substantial follow-up, unbiased towards certain antibody positivities in the absence of prescreening for ICA or enrichment in prediabetic relatives, as may be the case in some studies [4, 7], and with a wider age range than in other studies [6]. Our confirmation [13] of the primacy of IA-2A positivity over the number of autoantibodies—at variance with other claims [9]—should not be ascribed to differences in study size but rather to differences in study protocol and/or the way the data were analysed; indeed, when expressed in the same way as in other studies [4] our results also indicate an increasing risk of type 1 diabetes with the number of (molecular) antibodies present.
Both in IA-2A-positive and in IA-2A-negative patients with circulating diabetes autoantibodies, HLA DQ genotyping was found to provide additional information in terms of diabetes prediction. Cox regression analysis confirmed that the presence of DQ2/DQ8 complemented IA-2A positivity in the prediction model and overruled differences associated with the type of relationship and the number of antibodies. In IA-2A-positive relatives carrying DQ2/DQ8, progression to diabetes averaged 84% within 5 years, in line with the preferential association of DQ2/DQ8 with early-onset type 1 diabetes [3, 17, 18]; hence, simultaneous positivity for both markers can almost be considered a diagnostic criterion for diabetes long before glycaemia starts to rise, and defines a group of relatives at homogeneously high risk of type 1 diabetes. Carriers of DQ8 in combination with a haplotype other than DQ2 also tended to progress more rapidly to diabetes than DQ8-negative relatives, thereby confirming the preferential association of IA-2A and DQ8/DR4 in new-onset patients [31–33]. Recently, the strong diabetes-predictive value of IA-2A, especially in cases where there are high levels and specific subclasses, was independently confirmed in persistently antibody-positive relatives [34]. The use of combined positivity for IA-2A (above percentile 99) and HLA DQ2/DQ8 in one blood sample may, however, be more practical for the enrolment of high-risk subjects in prevention trials.
Future work should aim to increase the specificity of screening in relatives positive for other antibodies. In the absence of IA-2A, the presence of at least one other antibody type (IAA, ICA or GADA) did confer a significant—albeit low risk of diabetes. This risk increased with the number of antibodies present, as indicated by both survival analysis and Cox regression, and in carriers of HLA DQ2, regardless of DQ8 status, it is consistent with the reported preferential association of GADA with DQ2 in new-onset patients [31–33]. The differential HLA DQ enhancement of progression to diabetes according to IA-2A status supports previous suggestions that different diabetogenic pathways may converge to the same clinical endpoint of immune-mediated type 1 diabetes [35, 36]. Nevertheless, progression to diabetes in antibody-positive carriers of DQ2 remains low in the absence of IA-2A. Age, gender, BMI and 5′ INS genotype (K. Decochez, unpublished)—all known to influence the risk of diabetes [3, 18, 20, 22]—could not improve the prediction of diabetes so far in this group, but demonstration of their possible added value would require follow-up of larger study groups for longer periods. Disease prediction may further benefit from the assessment of the persistence of antibodies, changes in antibody levels and SDS-BMI over time and, most likely, from the repeated assessment of beta cell function and insulin resistance [22, 24, 37–42]. The most standardised functional tests (intravenous glucose tolerance test, clamps) are, however, more difficult to implement on a larger scale in non-diabetic risk groups. Since IA-2A can at present only be detected in about 70% of prediabetic subjects [13], prediction strategies would also benefit from an increase in sensitivity of IA-2A screening. In the present study the highly predictive combined presence of IA-2A and DQ2/DQ8 identifies only about 30% (13 out of 43) of the future diabetic relatives; therefore additional, e.g. functional, parameters would also be most welcome here to improve screening sensitivity.
In conclusion, (multiple) antibody positivity and progression to diabetes was graded according to the known hierarchy of risk among relatives, and confirmed our previous observation in siblings of the primacy of IA-2A positivity over multiple antibody positivity for the prediction of diabetes. Progression to diabetes was much more dependent on HLA DQ status than on the nature of kinship. Differences in the frequencies of HLA DQ risk genotypes could partly explain differences in the risk of diabetes between siblings and offspring, but not between offspring of a diabetic mother or father. In IA-2A-positive relatives there was significantly more progression to diabetes in the presence of DQ8, particularly when in heterozygous combination with DQ2; in the presence of islet antibodies different from IA-2A there was more progression in carriers of DQ2 regardless of DQ8 status. This differential HLA DQ enhancement according to IA-2A status may suggest the existence of at least two distinct pathogenic pathways for immune-mediated type 1 diabetes.
Abbreviations
- Ab:
-
antibody
- BDR:
-
Belgian Diabetes Registry
- DASP:
-
Diabetes Antibody Standardisation Program
- GADA:
-
glutamate decarboxylase antibodies
- IAA:
-
insulin autoantibodies
- IA-2A:
-
insulinoma-associated protein-2 antibodies
- ICA:
-
islet cell cytoplasmic antibodies
- IDW:
-
Immunology of Diabetes Workshops
- JDF:
-
Juvenile Diabetes Foundation
- SDS:
-
standard deviation score
References
Bingley PJ, Bonifacio E, Gale EAM (1993) Can we really predict diabetes? Diabetes 42:213–220
Slover R, Eisenbarth GS (1997) Prevention of type I diabetes and recurrent beta-cell destruction of transplanted islets. Endocr Rev 18:241–258
Gorus FK, Pipeleers DG, Belgian Diabetes Registry (2001) Prospects for predicting and stopping the development of type 1 diabetes. Best Pract Res Clin Endocrinol Metab 15:371–389
Verge CF, Gianani R, Kawasaki E et al (1996) Prediction of type 1 diabetes in first degree relatives using a combination of insulin, GAD and ICA512bdc/IA–2 autoantibodies. Diabetes 45:926–933
Gorus FK, Goubert PG, Semakula C et al (1997) IA-2-autoantibodies complement GAD65-autoantibodies in new-onset IDDM patients and help predict impending diabetes in their siblings. Diabetologia 40:95–99
Kulmala P, Savola K, Petersen JS et al (1998) Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. A population-based study. J Clin Invest 101:327–336
Bingley PJ, Christie MR, Bonifacio E et al (1994) Combined analysis of autoantibodies improves prediction of IDDM in islet-cell antibody positive relatives. Diabetes 43:1304–1310
Leslie RDG, Atkinson MA, Notkins AL (1999) Autoantigens IA-2 and GAD in type 1 (insulin-dependent) diabetes. Diabetologia 42:3–14
Maclaren N, Lan M, Coutant R et al (1999) Only multiple autoantibodies to islet cells (ICA), insulin, GAD65, IA-2 and IA-2β predict immune-mediated (type 1) diabetes in relatives. J Autoimmun 12:279–287
The European Nicotinamide Diabetes Intervention Trial (ENDIT) Group (2004) European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363:925–931
Diabetes Prevention Trial—Type 1 Study Group (2002) Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346:1685–1691
Mahon JL, Dupré J (1997) The limitations of clinical trials for prevention of IDDM. Diabetes Care 20:1027–1033
Decochez K, De Leeuw IH, Keymeulen B et al (2002) IA-2 autoantibodies predict impending type 1 diabetes in siblings of patients. Diabetologia 45:1658–1666
Yu L, Rewers M, Gianani R et al (1996) Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 81:4264–4267
Kupila A, Keskinen P, Simell T et al (2002) Genetic risk determines the emergence of diabetes-associated autoantibodies in young children. Diabetes 51:646–651
Ziegler AG, Hummel M, Schenker M et al (1999) Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes. The 2-year analysis of the German BABYDIAB Study. Diabetes 48:460–468
Caillat-Zucman S, Garchon HJ, Timsit J et al (1992) Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest 90:2242–2250
Van der Auwera B, Schuit F, Lyaruu I et al (1995) Genetic susceptibility for insulin-dependent diabetes mellitus in Caucasians revisited: the importance of diabetes registries in disclosing interactions between HLA DQ and insulin gene-limited risk. J Clin Endocrinol Metab 80:2567–2573
Warram JH, Krolewski AS, Gottlieb MS et al (1984) Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med 311:149–152
Gale EAM, Gillespie KM (2001) Diabetes and gender. Diabetologia 44:3–15
National Diabetes Data Group (1979) Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 28:1039–1057
Weets I, Van der Auwera BJ, Schuit FC et al (2001) Male-to-female excess in diabetes diagnosed in early adulthood is not specific for the immune-mediated form nor is it HLA-DQ restricted: possible relation to increased body mass index. Diabetologia 44:40–47
Cole TJ, Freeman JV, Preece MA (1995) Body mass index reference curves for the UK, 1990. Arch Dis Child 73:25–29
Decochez K, Tits J, Coolens JL et al (2000) High frequency of increasing islet-specific autoantibody levels after diagnosis of insulin-requiring type 1 diabetes presenting before age 40 years. Diabetes Care 23:838–844
Verge CF, Stenger D, Bonifacio E et al (1998) Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin, autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes. Diabetes 47:1857–1866
Bingley PJ, Bonifacio E, Mueller PW, participating laboratories (2003) Diabetes autoantibody standardization program: first assay proficiency evaluation. Diabetes 52:1128–1136
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170
Cox DR (1972) Regression models and life-tables. J R Stat Soc [B] 34:187–220
Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method. Br Med J 310:170
Genovese S, Bonfanti R, Bazzigaluppi E et al (1996) Association of IA-2 autoantibodies with HLA DR4 phenotypes in IDDM. Diabetologia 39:1223–1226
Vandewalle CL, Falorni A, Lernmark Å et al (1997) Associations of GAD65- and IA-2 autoantibodies with genetic risk markers in new-onset IDDM patients and their siblings. Diabetes Care 20:1547–1552
Graham J, Hagopian WA, Kockum I et al (2002) Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 51:1346–1355
Achenbach P, Warncke K, Reiter J et al (2004) Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 53:384–392
Cucca F, Goy JV, Kawaguchi Y et al (1998) A male–female bias in type 1 diabetes and linkage to chromosome Xp in MHC HLA-DR3-positive patients. Nat Genet 19:301–302
Ludvigsson J, Samuelsson U, De Beaufort C et al (1986) HLA-DR3 is associated with a more slowly progressive form of type 1 (insulin-dependent) diabetes. Diabetologia 29:207–210
Gorus FK, Vandewalle CL, Dorchy H et al (1994) Influence of age on the associations among insulin autoantibodies, islet cell antibodies and DQA1*0301-DQB1*0302 haplotype in siblings of IDDM patients. J Clin Endocrinol Metab 78:1172–1178
Yu J, Yu L, Bugawan TL et al (2000) Transient antiislet autoantibodies: infrequent occurrence and lack of association with ‘genetic’ risk factors. J Clin Endocrinol Metab 85:2421–2428
Landin-Olsson M, Arnqvist HJ, Blohmé G et al (1999) Appearance of islet cell autoantibodies after clinical diagnosis of diabetes mellitus. Autoimmunity 29:57–63
Srikanta S, Ganda OP, Rabizadeh A et al (1985) First-degree relatives of patients with type I diabetes mellitus. Islet-cell antibodies and abnormal insulin secretion. N Engl J Med 313:461–464
Røder ME, Knip M, Hartling SG et al (1994) Disproportionally elevated proinsulin levels precede the onset of insulin-dependent diabetes mellitus in siblings with low first phase insulin responses. J Clin Endocrinol Metab 79:1570–1575
Elahi D (1996) In praise of the hyperglycaemic clamp. A method for assessment of β-cell sensitivity and insulin resistance. Diabetes Care 19:278–286
Acknowledgements
The present work was supported by the Belgian Fund for Scientific Research (Dutch-speaking branch: research grants 7-0021-96, 3-0113-97, G-0319-01 and G-0517-04, research fellowships to K.D. and I.T. and postdoctoral fellowships to C.M. and I.W.; French-speaking branch: research grants 3-4525-96 and 3-4531-97) and the research council of the Brussels Free University–VUB (a research fellowship to E.V.). The Belgian Diabetes Registry is supported financially by the Belgian National Lottery, the Ministries of Public Health of the Flemish and French Communities of Belgium, Weightwatchers, Ortho Clinical Diagnostics, Novo Nordisk, Lifescan, Roche Diagnostics and Bayer.
The expert technical assistance of the co-workers at the central unit of the Belgian Diabetes Registry (V. Claessens, A. Demarré, L. De Pree, S. Exterbille, P. Goubert, C. Groven, A. Ivens, D. Kesler, F. Lebleu, E. Quartier, G. Schoonjans, H. Thomas, M. Van der Linden and S. Vanderstraeten) is gratefully acknowledged. We would also like to thank the different university teams of co-workers for their excellent assistance in collecting samples and organising the fieldwork in Antwerp (J. Michiels and J. Vertommen), Brussels (N. Alaerts, M. Bodson, T. De Mesmaeker and T. Ghysels), Ghent (N. Christophe, E. De Man, S. De Neve, A. Hutse) and Leuven (M. Carpentier, A. Ceusters, C. Lauwers, H. Morobé). We are indebted to Professor L. Kaufman (Department of Biostatistics, Brussels Free University–VUB) for his invaluable statistical advice.
We sincerely thank all members of the Belgian Diabetes Registry who contributed to the recruitment of relatives for the present study, but cannot be listed individually because of space limitations.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
K. Decochez and I. Truyen contributed equally to the present work.
Rights and permissions
About this article
Cite this article
Decochez, K., Truyen, I., Van der Auwera, B. et al. Combined positivity for HLA DQ2/DQ8 and IA-2 antibodies defines population at high risk of developing type 1 diabetes. Diabetologia 48, 687–694 (2005). https://doi.org/10.1007/s00125-005-1702-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1702-x